How To Find Neutrons In A Periodic Table
Questions and Answers
How many protons, electrons and neutrons are in an cantlet of krypton, carbon, oxygen, neon, argent, gold, etc...?
To find the number of protons, electrons and neutrons in an atom, just follow these easy steps:
Footstep i - Gather Information
The showtime thing you lot volition demand to do is find some information virtually your element. Go to the Periodic Table of Elements and click on your chemical element. If information technology makes things easier, yous can select your element from an alphabetical listing.
Use the Table of Elements to find your chemical element's diminutive number and atomic weight. The atomic number is the number located in the upper left corner and the atomic weight is the number located on the bottom, equally in this example for krypton:
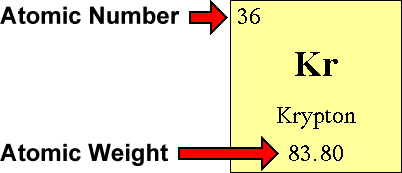
Step ii - The Number of Protons is...
The diminutive number is the number of protons in an cantlet of an chemical element. In our example, krypton's atomic number is 36. This tells us that an cantlet of krypton has 36 protons in its nucleus.
The interesting matter here is that every atom of krypton contains 36 protons. If an atom doesn't have 36 protons, it can't be an atom of krypton. Adding or removing protons from the nucleus of an atom creates a dissimilar element. For example, removing i proton from an cantlet of krypton creates an atom of bromine.
Pace 3 - The Number of Electrons is...
Past definition, atoms have no overall electrical charge. That means that there must exist a residuum between the positively charged protons and the negatively charged electrons. Atoms must have equal numbers of protons and electrons. In our case, an cantlet of krypton must contain 36 electrons since it contains 36 protons.
Electrons are bundled effectually atoms in a special fashion. If you need to know how the electrons are bundled around an atom, accept a look at the 'How do I read an electron configuration table?' page.
An atom can gain or lose electrons, becoming what is known as an ion. An ion is nothing more than an electrically charged cantlet. Adding or removing electrons from an atom does not modify which element it is, just its internet charge.
For example, removing an electron from an atom of krypton forms a krypton ion, which is unremarkably written every bit Kr+. The plus sign ways that this is a positively charged ion. It is positively charged considering a negatively charged electron was removed from the cantlet. The 35 remaining electrons were outnumbered past the 36 positively charged protons, resulting in a charge of +ane.
Step 4 - The Number of Neutrons is...
The atomic weight is basically a measurement of the full number of particles in an cantlet'due south nucleus. In reality, it isn't that make clean cut. The atomic weight is actually a weighted boilerplate of all of the naturally occurring isotopes of an chemical element relative to the mass of carbon-12. Didn't understand that? Doesn't thing. All you really need to notice is something called the mass number. Unfortunately, the mass number isn't listed on the Table of Elements. Happily, to discover the mass number, all yous demand to practise is round the atomic weight to the nearest whole number. In our case, krypton's mass number is 84 since its atomic weight, 83.80, rounds upward to 84.
The mass number is a count of the number of particles in an atom'due south nucleus. Remember that the nucleus is fabricated up of protons and neutrons. And so, if we want, we can write:
Mass Number = (Number of Protons) + (Number of Neutrons)
For krypton, this equation becomes:
84 = (Number of Protons) + (Number of Neutrons)
If we only knew how many protons krypton has, we could effigy out how many neutrons it has. Wait a minute... Nosotros do know how many protons krypton has! We did that back in Step 2! The atomic number (36) is the number of protons in krypton. Putting this into the equation, we go:
84 = 36 + (Number of Neutrons)
What number added to 36 makes 84? Hopefully, you said 48. That is the number of neutrons in an cantlet of krypton.
The interesting affair here is that adding or removing neutrons from an atom does not create a different element. Rather, it creates a heavier or lighter version of that element. These unlike versions are chosen isotopes and almost elements are actually a mixture of different isotopes.
If you lot could take hold of atoms of krypton and count the number of neutrons each one had, you would find that most would accept 48, others would have 47, some would have l, some others would have 46, a few would have 44 and a very few would have 42. You would count unlike numbers of neutrons because krypton is a mixture of six isotopes.
In Summary...
For any element:
Number of Protons = Diminutive Number
Number of Electrons = Number of Protons = Atomic Number
Number of Neutrons = Mass Number - Diminutive Number
For krypton:
Number of Protons = Atomic Number = 36
Number of Electrons = Number of Protons = Diminutive Number = 36
Number of Neutrons = Mass Number - Atomic Number = 84 - 36 = 48
Related Pages:
How To Find Neutrons In A Periodic Table,
Source: https://education.jlab.org/qa/pen_number.html
Posted by: yockeybegry1954.blogspot.com


0 Response to "How To Find Neutrons In A Periodic Table"
Post a Comment